Why should you ask your doctor about VALSTAR®?
VALSTAR® is an FDA-approved treatment option for your carcinoma in situ (CIS) bladder cancer that failed previous therapies and if you’re not a candidate for surgery.
Find your reasons
-


Prior treatment

Prior treatment
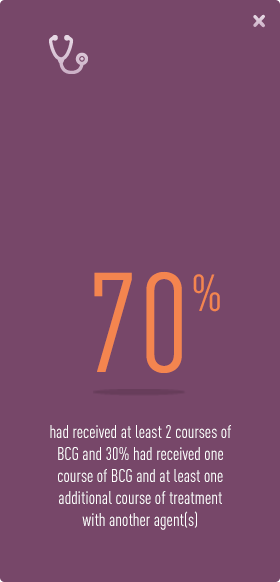
Your CIS bladder cancer has already failed prior treatments
The clinical trials for VALSTAR® were conducted in 90 patients with CIS bladder cancer, all of whom had failed to respond to one, or more, prior treatments.
-


Treatment with VALSTAR®


Treatment with VALSTAR®
16 of 90 patients (18%) no longer had CIS at 6 months after treatment with VALSTAR®.

During the clinical trials, 16 of the 90 patients (about 1 in 5) did not have CIS at 6 months after completing treatment with VALSTAR®.
-


Duration of response


Duration of response
no longer had CIS at 6 months after treatment with VALSTAR® and did not have a recurrence for more than 1 year from the start of treatment.
Prior to starting VALSTAR®, treatments may have included a procedure to remove all or part of the CIS bladder cancer tumor and may have been followed by BCG therapy.
-


Time to recurrence


Time to recurrence
Time to recurrence was longer after treatment with VALSTAR® compared to previous courses of intravesical therapy.
In a retrospective analysis of the 16 patients who did not have CIS at 6 months, the time before their cancer tumors returned was longer after treatment with VALSTAR® compared with previous therapies.
-


Safety with VALSTAR®


Safety with VALSTAR®
Only about 1 in 5 patients no longer had CIS bladder cancer 6 months after treatment with VALSTAR®. Waiting to have your bladder taken out could lead to the spread of bladder cancer and even death. In a study of VALSTAR®, 10 out of the 90 patients developed deeply invasive bladder cancer; 4 of them died with metastatic bladder cancer and 6 had progressed to a deeply invasive disease by the time they had surgery to remove their bladder. You should talk with your doctor about the risks of waiting for surgery to have your bladder taken out when you have CIS bladder cancer.
-


Common side effects of treatment with VALSTAR®


Common side effects of treatment
with VALSTAR®- urinating often
- difficulty urinating
- a strong need to urinate right away
- bladder spasm
- burning and pain
- blood in urine
- not making it to the bathroom in time to urinate
- bladder inflammation
- urinary tract infection
- need to urinate at night
- stomach pain
- nausea
These are not all the side effects of VALSTAR®. For more information, ask your doctor.

